Since May 2020, when Chinese President Xi Jinping announced at a World Health Assembly meeting that China considered its COVID-19 vaccines to be a “global public good,” China has been busy offering its products to the world. But it does not necessarily follow that Chinese vaccines would be provided for free. Even less so does China treat its customers equally. Some countries received vaccines in the form of donations, while others purchased them or were offered a loan to buy them – an alternative aimed primarily at the Latin American and Caribbean countries.
The situation is akin to spring 2020, when the outbreak of the pandemic and the lack of protective medical equipment prompted many countries to court China. Already during the first wave of the coronavirus epidemic, China attempted to improve its image through “mask diplomacy,” posing as a part of the solution, rather than the source of the problem. “Vaccine diplomacy” can be understood as a natural extension of this process.
However, unlike last spring, China is no longer the sole provider of a scarce resource. Instead, China is having to compete with various other vaccine producers. In addition, the producers have to deal with “borders” erected by medical regulatory institutions as the requirements for vaccines are stricter in comparison to regulations on masks and other protective material. In order not to clash directly with the mRNA vaccines produced by Pfizer/BioNTech or Moderna, bound mostly for developed countries, Chinese vaccines based on inactivated virus were first offered to its neighbors, the developing world, and countries in the semi-peripheries.
The comprehensive table below, based on open-source information, provides a glimpse at the logic of China’s “vaccine diplomacy.” It shows that China has donated its vaccines to countries in quantities of tens of thousands of doses. Provision of a “free sample” often resulted in the recipient country’s interest in the purchase of the vaccine, this time in millions of doses. What is worth noticing is that China’s donations come almost exclusively in the form of vaccines produced by Sinopharm, outshining the other two internationally known Chinese vaccines, CoronaVac (produced by Sinovac) and Conviecia (manufactured by CanSino Biologics).
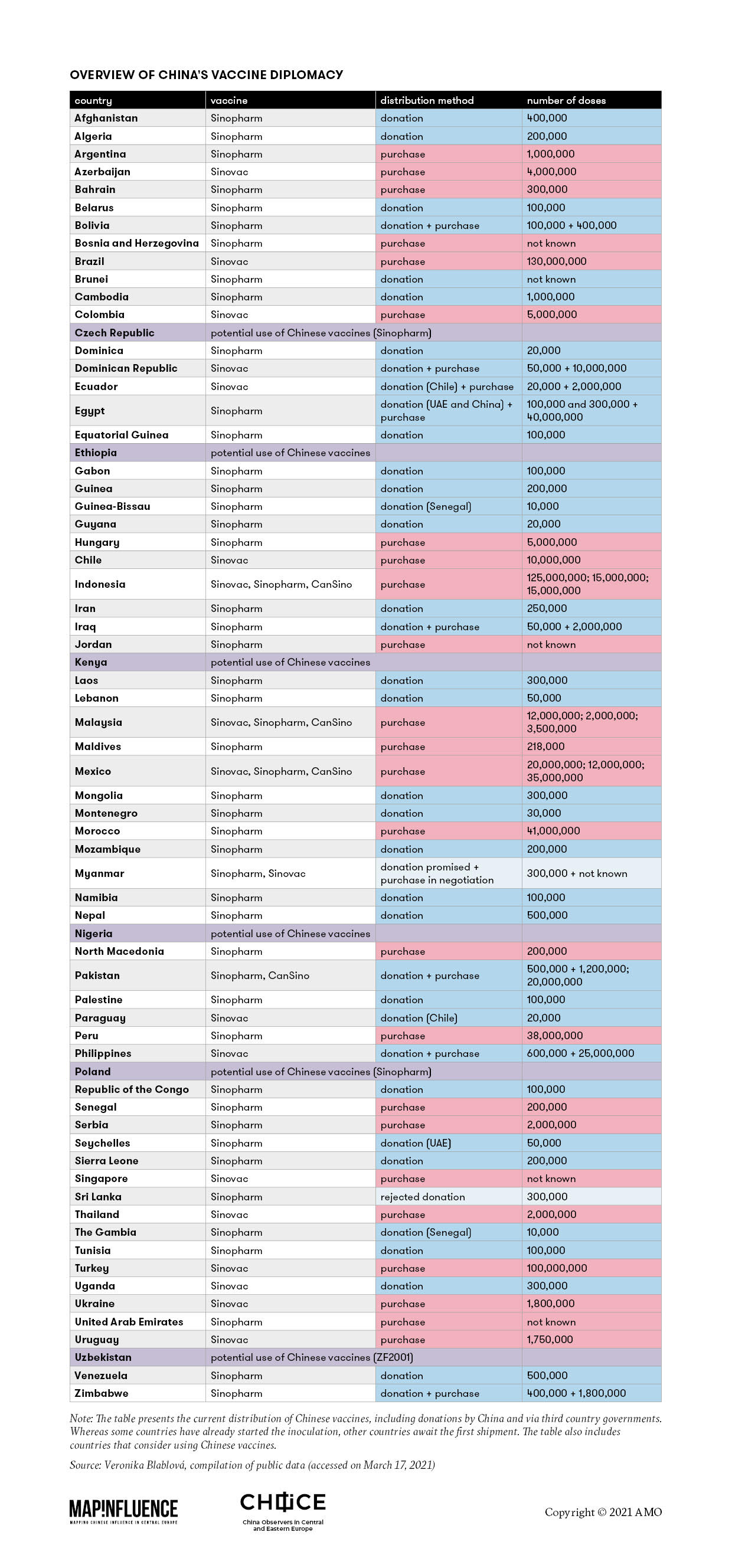
The table is reprinted with the permission of the copyright holder, the Association for International Affairs (AMO).
Chinese Vaccine Geography
In Asia, China has been busy securing vaccine markets close to home. Nine out of the 10 ASEAN countries (with the exception of Vietnam) are on track to use Chinese vaccines. To Brunei, Cambodia, Laos, and Myanmar, China promised vaccines in the form of a donation. The Philippines received a donation, which later resulted in Manila’s purchase of the vaccines, while Indonesia, Malaysia, Singapore, and Thailand bought Chinese vaccines straight away.
In other Asian countries, however, China has often clashed with Indian vaccine diplomacy, as evident in the case of Sri Lanka. A Chinese donation has been put on hold due to a lack of documentation, while India went ahead with its donation. A similar scenario may be identified in Nepal, with both China and India offering to donate jabs. But it was, again, India that later provided additional vaccines. Bangladesh also opted for Indian vaccines after it refused to co-fund Chinese vaccine trials.
Chinese vaccines have found their way to other continents. In South America, China managed to effectively introduce its vaccines to all countries apart from Suriname and French Guyana. The fact that Sinovac held vaccine trials in Brazil contributed to the enhanced credibility of the Chinese vaccine in the region, despite the fact that trials showed that the vaccine had lower efficacy than leading alternatives. The majority of countries accepted donations or ordered Sinovac, with only Argentina and Bolivia choosing to inoculate their populations with Sinopharm. The Chinese position within the region may be even more consolidated in the future as Brazil intends to augment the domestic production of Sinovac.
On the other hand, Paraguay demonstrates the political incentives behind Chinese “vaccine diplomacy.” As one of 15 remaining countries that diplomatically recognizes Taiwan, Paraguay accessed Sinovac only through a donation from neighboring Chile. In April 2020, Paraguayan senators discussed opening relations with China amid a lack of masks and medical protective material. This proposal was eventually rejected but the discussion was launched and it may appear on the table again if and when the opposition parties prevail.
In Central America, Mexico acts as one of the largest “Chinese vaccine hubs,” offering the whole spectrum of the currently distributed Chinese vaccines. In comparison, however, Indian vaccine diplomacy dominates the region. Moreover, a number of Caribbean and Central American countries recognize Taiwan diplomatically, which complicates the involvement of Chinese vaccines.
Besides Latin America, the Chinese effort has further concentrated on the Middle East and North Africa, aiming to strengthen relations. The United Arab Emirates was the first country outside China to approve Sinopharm’s vaccine and actively participated in the third phase of the clinical tests. It has also signed a contract to manufacture the vaccine, so the situation is akin to developments in South America.
Concerning sub-Saharan Africa, Chinese vaccines seem to be predominantly distributed through the COVAX initiative. Based on the data collected, India seems ahead of China as a vaccine supplier to the region. Nonetheless, China endeavors not to lag behind and has already donated several hundred thousand Sinopharm doses, for instance to Zimbabwe, Mozambique, and Namibia.
Entering Europe
Four factors usually inform the decision of countries on vaccine purchases: price, capacity to deliver a sufficient amount, safety, and efficacy. In all these areas, however, questions persist regarding the Chinese vaccines. As far as price is concerned, the data are scarce, with a few notable examples reaching the public indicating that the price of the vaccine produced by Sinopharm is anything but low. There are also questions regarding Chinese capacities to deliver a sufficient amount of doses, especially if China decides to prioritize its vast domestic population. In order for a vaccine to be administered, it must be marked as safe and effective, which in the European Union, for example, means being approved by the European Medicines Agency (EMA). However, as the approval process is time-consuming, national medical regulators may authorize vaccines for emergency use. As a result, and despite the continuing lack of information on Chinese vaccines as well as their widely varying efficacy rates in testing abroad (ranging, in the case of Sinopharm, from 91 percent in Turkey to 50.4 percent in Brazil), some countries have already approved Chinese vaccines for emergency use.
Nowhere has the debate been so heated as in Europe, where – despite the fact that none of China’s vaccine manufacturers has yet applied for approval to the EMA – some Central and Eastern European countries have been busy asking for the Chinese jabs. Serbia has already begun vaccinating its population with Sinopharm’s vaccine, while Belarus, Bosnia and Herzegovina, Montenegro, North Macedonia, and Ukraine are believed to be on track to use Chinese vaccines as well. Among the EU member states, Hungary has been using Sinopharm, Czechia has asked Beijing for the delivery of the same vaccine, and Poland has been considering it. While ostensibly looking for a “silver bullet” to end the epidemic, the steps of the governments of Central and Eastern Europe risk opening a new window of opportunity for China’s influence in the region.
In a number of countries, the overall narrative connected to the Chinese vaccines is that vaccines know no politics. Unlike the case of letting Chinese vendors build up 5G networks, or allowing companies to participate in critical energy infrastructure, vaccines allegedly do not pose any security risk. The logic of vaccine diplomacy, however, shows that China approaches the issue not only from a business perspective, but also taking into account political incentives. Jabs are used as a tool, to reinforce established relations and capitalize on new opportunities.